Phosphorus, a reactive non-metal element, holds a significant position in numerous fields of science and industry. Its versatile nature arises from its unique chemical properties, directly influenced by the number of valence electrons it possesses. Embark on a journey to unravel the secrets of phosphorus, as we delve into the captivating realm of valence electrons, their configuration, and the profound impact they have on phosphorus’s behavior.
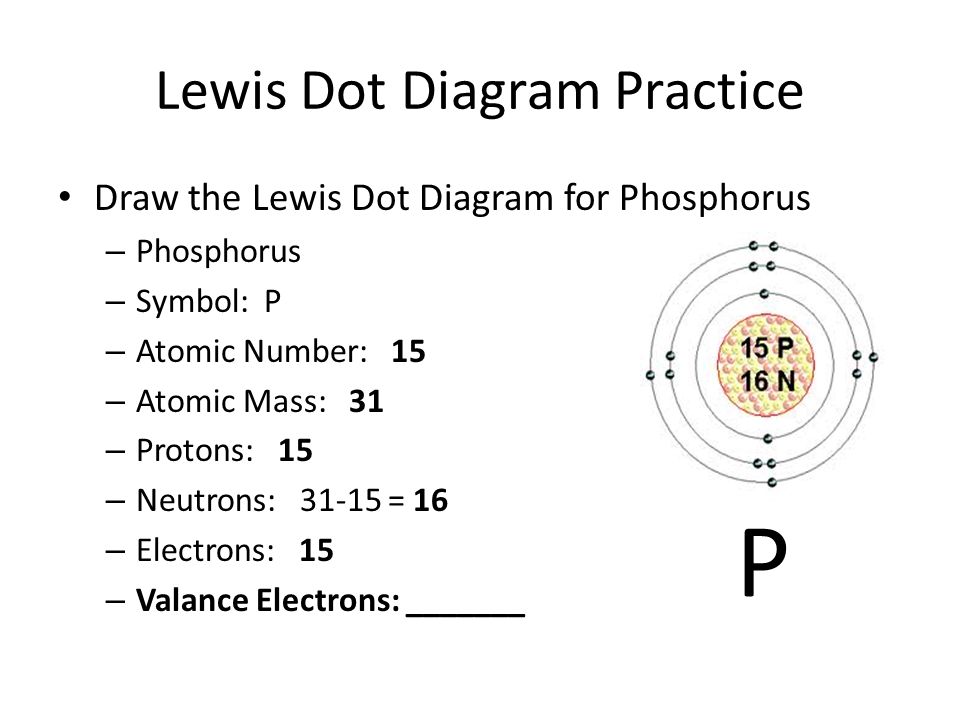
Image: periodictable.me
Phosphorus, denoted by the symbol ‘P’, ranks 15th on the Periodic Table of Elements, nestled within Group 15, also known as the nitrogen family. This placement hints at its non-metallic characteristics, evident in its solid form at room temperature and its eager participation in chemical reactions. Phosphorus finds applications in diverse industries, ranging from fertilizer production to detergent manufacturing.
Exploring the Realm of Valence Electrons
Valence electrons, often referred to as the workhorses of chemical reactions, occupy the outermost energy level of an atom, dictating its chemical reactivity. They partake in the formation and breaking of chemical bonds, profoundly influencing an element’s behavior and properties. Phosphorus, being a member of Group 15, boasts five valence electrons. These electrons reside in the third energy level, eagerly awaiting the chance to engage in chemical interactions.
Phosphorus exhibits various oxidation states, further emphasizing the significance of its valence electrons. The most common oxidation states for phosphorus are +3, +4, and +5, reflecting the number of valence electrons it can share, accept, or donate in chemical reactions. The versatility of phosphorus’s valence electrons enables it to form a wide range of compounds, contributing to its diverse applications.
Phosphorus’s Electronic Configuration: A Deeper Dive
Delving into the electronic configuration of phosphorus unveils a more nuanced understanding of its valence electrons. The electronic configuration of phosphorus is 1s22s22p63s23p3. This configuration depicts the distribution of electrons across different energy levels and orbitals. The three valence electrons reside in the 3p subshell, poised to engage in chemical bonding.
The 3p subshell consists of three orbitals, namely 3px, 3py, and 3pz. Each of these orbitals can accommodate a maximum of two electrons, giving rise to the six possible valence electrons for phosphorus. However, phosphorus has only three valence electrons, resulting in the electronic configuration 3p3.
Hybridization of Valence Electrons: Unleashing Versatility
Phosphorus’s valence electrons not only determine its reactivity but also play a crucial role in its molecular geometry. Hybridization, a concept that combines atomic orbitals to form new hybrid orbitals, profoundly influences the shape and properties of molecules. Phosphorus undergoes two primary types of hybridization: sp3 and sp3d.
In sp3 hybridization, one s orbital and three p orbitals combine to form four equivalent hybrid orbitals, each with a tetrahedral geometry. This hybridization gives rise to molecules with a tetrahedral shape, such as phosphorus trichloride (PCl3). In contrast, sp3d hybridization involves the combination of one s orbital, three p orbitals, and one d orbital, resulting in five hybrid orbitals with a trigonal bipyramidal geometry. Phosphorus pentachloride (PCl5) exemplifies a molecule with sp3d hybridization, exhibiting a trigonal bipyramidal shape.
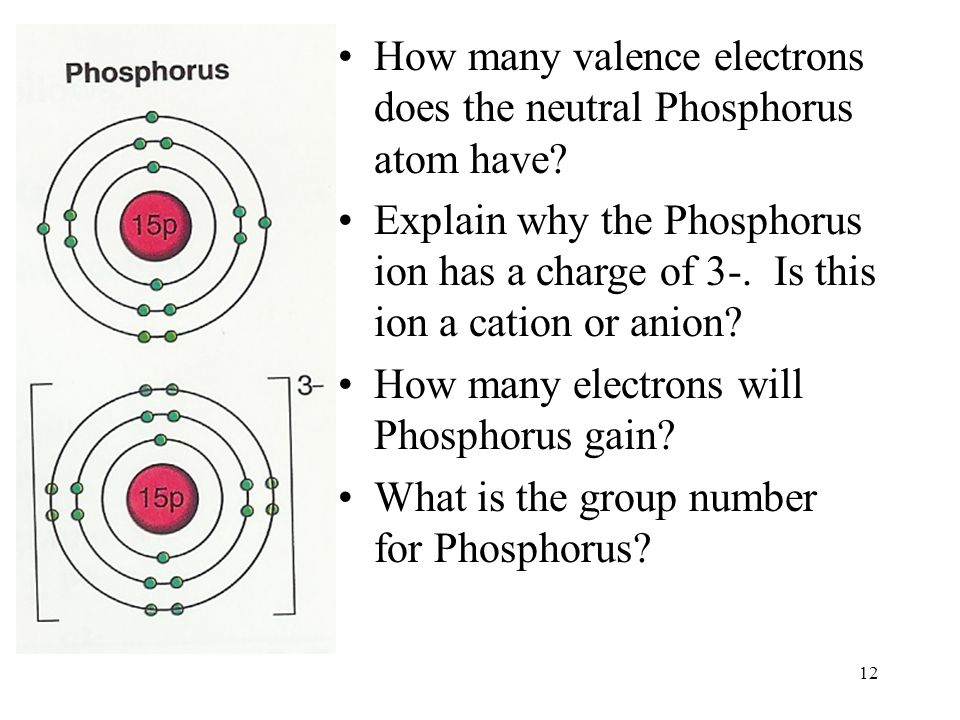
Image: periodictable.me
Phosphorus in Action: Applications Driven by Valence Electrons
Phosphorus’s unique valence electron configuration and versatility have propelled its use in a plethora of applications. Its involvement in the production of fertilizers, such as ammonium phosphate and superphosphate, is paramount in supporting global agriculture and feeding the world’s population. Phosphorus also plays a significant role in detergent formulations, enhancing their ability to remove dirt and stains from fabrics.
Moreover, phosphorus finds applications in the production of safety matches, where its role in the ignition process is crucial. Its presence in fireworks contributes to the captivating colors and pyrotechnic effects that illuminate night skies during celebrations. The pharmaceutical industry also utilizes phosphorus in the synthesis of vitamins, antibiotics, and other essential drugs.
How Many Valence Electrons Does P Have
Conclusion
Unveiling the captivating world of phosphorus and its valence electrons unveils a treasure trove of chemical knowledge. Phosphorus’s position in Group 15 of the Periodic Table, with its five valence electrons, empowers it with remarkable versatility. These valence electrons dance across energy levels, influencing oxidation states and participating in hybridization, ultimately shaping the element’s reactivity, molecular geometry, and wide-ranging applications. From fertilizers to pharmaceuticals, the presence of phosphorus and its valence electrons touches countless aspects of our daily lives, underscoring the profound influence of chemistry in our modern world.