Introduction: The Chemistry of Carbon, the Building Block of Life
Carbon, the versatile and abundant element, weaves the fabric of countless molecules that sustain life on Earth. From the intricate structure of DNA to the vibrant colors of plant pigments, carbon’s influence is inescapable. One such carbon-based compound, butane, plays a crucial role in our daily lives as a fuel and chemical feedstock. In this article, we embark on a journey to uncover the number of carbon atoms concealed within a modest quantity of butane, unraveling a captivating tale of chemical analysis and scientific principles.
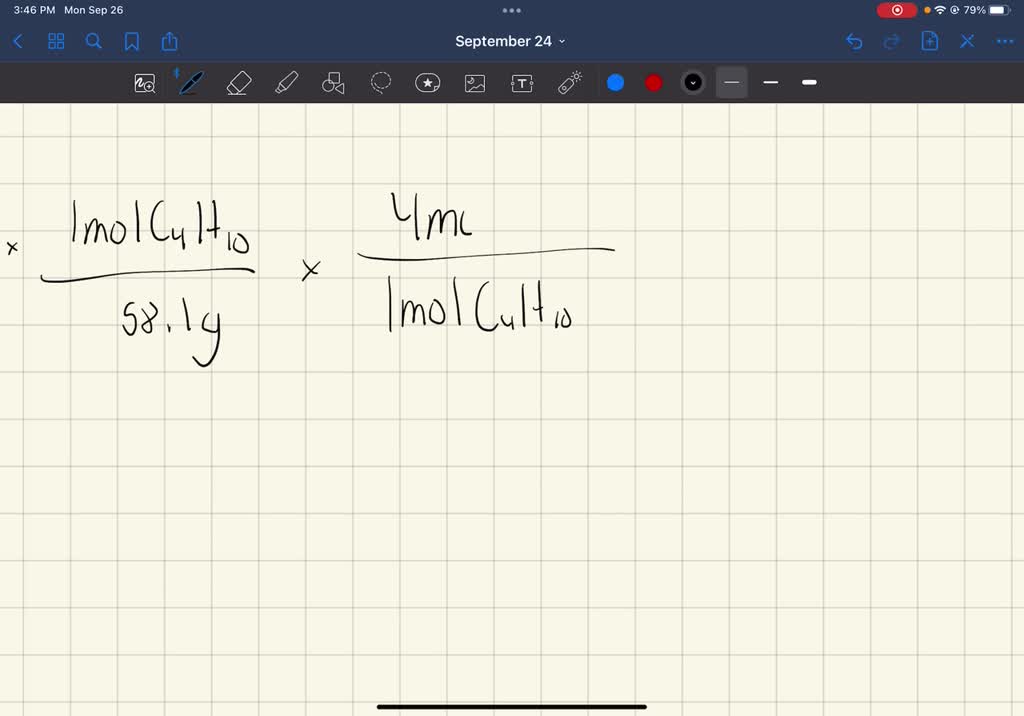
Image: www.numerade.com
Delving into the Chemical Makeup of Butane
Butane, a hydrocarbon with the molecular formula C4H10, belongs to the alkane family of compounds. These molecules consist solely of carbon and hydrogen atoms, arranged in a simple yet elegant manner. Each carbon atom in the chain-like structure bonds to four other atoms, forming a stable molecular framework. Understanding the composition of butane paves the way for determining the number of carbon atoms in a specific sample.
Calculating the Carbon Count: A Triumph of Chemical Arithmetic
To embark on this calculation, we must summon the principles of stoichiometry, the branch of chemistry that deciphers the quantitative relationships within chemical reactions. Begin by converting the mass of butane into the number of moles. Using the molar mass of butane (58.12 g/mol), we find that 4.00 g of butane corresponds to 0.0688 moles.
Since butane contains four carbon atoms per molecule, we simply multiply the number of moles of butane by the number of carbon atoms in each molecule:
Number of carbon atoms = Number of moles of butane × Number of carbon atoms per molecule of butane
Number of carbon atoms = 0.0688 mol × 4
Number of carbon atoms = 2.75 × 1023
Decoding the Number: A Vast Multitude of Carbon Atoms
A staggering 2.75 × 1023 carbon atoms reside within a mere 4.00 g of butane. This astronomical number underscores the extraordinary abundance of carbon in the universe and its pivotal role in the intricate chemical symphony of life. Butane itself finds widespread application in various industries, serving as a fuel for lighters, camping stoves, and even barbecues. Its chemical versatility extends to its use as a feedstock for the production of plastics, synthetic rubber, and numerous other products that shape our modern world.
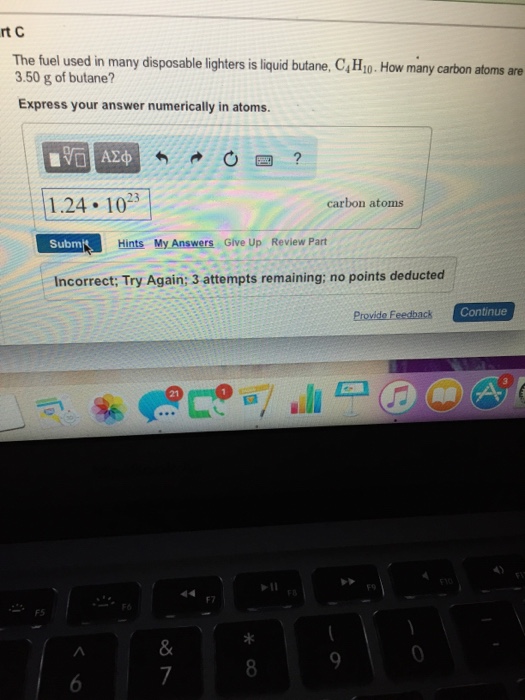
Image: www.chegg.com
Beyond Calculation: The Implications of Carbon Count
While the sheer number of carbon atoms in butane may seem abstract at first, its ramifications are profound. The understanding of stoichiometry empowers chemists and scientists to decipher the quantitative relationships in countless chemical reactions. From optimizing industrial processes to designing new drugs, precise knowledge of molecular composition is an invaluable asset in the pursuit of scientific advancements.
Expert Insights: The Perspectives of Seasoned Chemists
“The accurate determination of the number of carbon atoms in a substance is an essential skill for chemists,” remarks Dr. Emily Carter, a renowned professor of chemistry at Princeton University. “Stoichiometry provides a powerful tool for deciphering the composition of matter, enabling us to understand chemical reactions and develop new materials.”
Dr. Michael Faraday, a pioneer in the field of electrochemistry, once said, “The knowledge of the number of atoms in a given weight of a substance is a knowledge essential to us.” This quote beautifully captures the enduring importance of stoichiometry and its significance in unraveling the secrets of the chemical world.
Actionable Tips: Leveraging Stoichiometric Truths
-
Master mole conversion: Grasping the principles of converting between mass and moles is key to understanding stoichiometry. This skill empowers you to effortlessly translate between the macroscopic and microscopic realms of Chemistry.
-
Practice solving stoichiometry problems: Active engagement in solving stoichiometry problems strengthens your problem-solving abilities and deepens your understanding of chemical calculations.
-
Seek expert guidance: If you encounter difficulties in understanding stoichiometry, don’t hesitate to seek assistance from your instructors, peers, or online resources. With dedication and perseverance, you can conquer the intricacies of this captivating field.
How Many Carbon Atoms Are In 4.00 G Of Butane
Conclusion: The Power of Understanding, the Limitless Potential of Chemistry
Our journey to determine the number of carbon atoms in 4.00 g of butane has illuminated the power of stoichiometry and its significance in unraveling the mysteries of the molecular world. Whether it’s the development of innovative technologies or the optimization of industrial processes, the understanding of stoichiometry provides a solid foundation for scientific exploration. As we delve deeper into the wondrous realm of Chemistry, the knowledge gleaned from this endeavor will continue to empower our quest for scientific enlightenment.