Baking soda, an indispensable ingredient in the realm of baking, finds its place in countless kitchens across the globe. This unassuming substance holds a remarkable molecular structure that bestows upon it distinctive chemical properties. Understanding the intricacies of baking soda’s molecular mass is crucial in appreciating its significance in culinary and cleaning applications.

Image: ar.inspiredpencil.com
At the core of baking soda lies its chemical formula: NaHCO3. This notation reveals that a single molecule of baking soda consists of one sodium atom (Na), one hydrogen atom (H), one carbon atom (C), and three oxygen atoms (O). The atomic mass, representing the weight of a single atom, serves as the basis for determining the molecular mass of baking soda. The atomic mass of sodium is approximately 22.99 amu (atomic mass unit), hydrogen’s atomic mass is around 1.008 amu, carbon’s is approximately 12.011 amu, and oxygen’s atomic mass is around 15.9994 amu.
To ascertain the molecular mass of baking soda, we multiply the atomic mass of each element by its corresponding number of atoms in the molecule and then add these individual masses together. Applying this approach to baking soda, we obtain the molecular mass: (22.99 amu x 1) + (1.008 amu x 1) + (12.011 amu x 1) + (15.9994 amu x 3) = 84.0068 amu. Therefore, the molecular mass of baking soda is approximately 84.007 amu.
Recognizing the molecular makeup of baking soda is fundamental to comprehending its chemical behavior. The molecular mass, representing the combined weight of its constituent atoms, plays a vital role in determining substance properties, reactivity with other compounds, and its overall physical characteristics. In the context of baking, the molecular mass influences the rate at which baking soda reacts with acidic ingredients, releasing carbon dioxide gas that drives the leavening process in baked goods. Understanding baking soda’s molecular mass also aids in determining the appropriate量的additive for desired outcomes, ensuring consistent results in culinary endeavors.
Delving further into its molecular realm, the bicarbonate ion (HCO3–) in baking soda merits attention. Unlike the neutral nature of other salts, such as sodium chloride (NaCl), baking soda exhibits a mild alkaline property due to the presence of the bicarbonate ion. This inherent alkalinity bestows upon baking soda its exceptional cleaning and odor-neutralizing capacities, making it a versatile household staple. Understanding the molecular mass of baking soda enables effective utilization of its multifaceted properties, from culinary adventures to domestic cleaning challenges.
Baking Soda’s Culinary Contributions
In the world of baking, baking soda reigns supreme as a leavening agent, indispensable for creating delectable pastries, cakes, and other baked treats. Its molecular mass influences the reaction rate with acidic ingredients, guaranteeing optimal leavening. When combined with an acidic component, such as buttermilk, yogurt, or lemon juice, the reaction of baking soda with vinegar produces carbon dioxide gas, leading to the formation of bubbles within the batter. This gas expansion causes the batter to rise, resulting in airy, light-textured baked goods that tantalize taste buds and evoke admiration.
Beyond baked creations, baking soda’s versatility extends to savory culinary endeavors. Its alkaline nature lends itself to tenderizing tough meats, making it a valuable ally in preparing succulent, mouthwatering dishes. Moreover, baking soda contributes to browning reactions, adding a delightful golden-brown hue to baked goods and enhancing their overall visual appeal. Understanding the role of baking soda’s molecular mass in these processes empowers home cooks and bakers to achieve extraordinary culinary feats.
Unveiling Baking Soda’s Household Prowess
Baking soda, with its remarkable molecular structure, extends its utility beyond culinary endeavors, emerging as a formidable household ally. It possesses exceptional cleaning and odor-neutralizing abilities, making it a versatile cleaning agent for various surfaces. The alkaline nature imparted by its bicarbonate ion allows baking soda to effectively break down dirt, grease, and stains, restoring surfaces to their pristine condition. Its mild abrasive properties gently scrub away grime, without causing damage to delicate surfaces.
Harnessing the power of baking soda’s molecular mass in cleaning applications reveals its remarkable versatility. In laundry, baking soda acts as a natural whitener and odor remover, breathing new life into fabrics. Its ability to neutralize acidic odors makes it an effective agent for combating unpleasant smells in refrigerators, carpets, and other enclosed spaces. Understanding the molecular makeup of baking soda empowers individuals to create their own effective, eco-friendly cleaning solutions, promoting a healthy and fresh home environment.
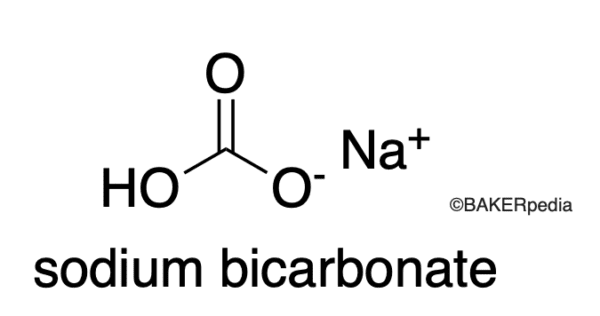
Image: bakerpedia.com
What Is The Molecular Mass Of Baking Soda
Conclusion
Comprehending the molecular mass of baking soda unlocks a wealth of knowledge about this humble yet versatile substance. Its unique molecular structure, consisting of sodium, hydrogen, carbon, and oxygen atoms, bestows upon baking soda distinctive chemical properties that find application in culinary and cleaning realms. In baking, the molecular mass influences the rate of reaction with acidic ingredients, driving the leavening process that creates airy, delectable baked goods. In cleaning, the alkaline nature imparted by its bicarbonate ion empowers baking soda to tackle dirt, grease, and odors, leaving surfaces refreshed and revitalized.