Sugars, the essential energy source for all living organisms, come in various forms and possess unique characteristics. Among them, reducing and non-reducing sugars stand out as two distinct categories, each with its own set of properties and biochemical significance. Understanding the difference between these two types of sugars is crucial in fields like food chemistry, nutrition, and medicine.
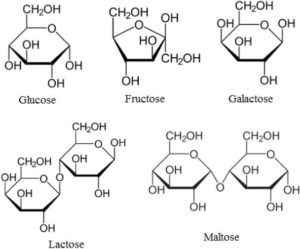
Image: americangardener.net
Distinguishing Between Reducing and Non-Reducing Sugars
The key factor that differentiates reducing sugars from non-reducing sugars lies in their structure. Reducing sugars possess a free aldehyde group (-CHO) or a free keto group (=O) in their molecular structure, while non-reducing sugars lack these groups. This structural difference endows reducing sugars with the ability to undergo redox reactions, where they can donate electrons to certain oxidizing agents such as Benedict’s solution or Fehling’s solution.
The presence of a free aldehyde or keto group in reducing sugars allows them to react with these oxidizing agents, reducing them to copper(I) oxide (Cu2O) in the case of Benedict’s solution or cuprous oxide (Cu2O) in the case of Fehling’s solution. This reaction is evident by the formation of a brick-red precipitate, indicating the presence of reducing sugars. Non-reducing sugars, on the other hand, do not contain these reactive functional groups and therefore do not undergo this redox reaction, hence their designation as non-reducing sugars.
Classifying Monosaccharides and Disaccharides
Among the simplest sugars, called monosaccharides, glucose, fructose, and galactose are examples of reducing sugars due to their free aldehyde or keto groups. Conversely, non-reducing monosaccharides include ribulose and xylose, which lack such functional groups. When two monosaccharides combine to form a disaccharide, the resulting sugar may be a reducing sugar or a non-reducing sugar depending on whether it contains a free aldehyde or keto group. For instance, lactose, a disaccharide found in milk, is a reducing sugar because it contains a free aldehyde group, while sucrose, the common table sugar, is a non-reducing sugar due to the absence of this functional group.
Industrial and Biological Roles
The reducing properties of reducing sugars find applications in various industries. The redox reaction between reducing sugars and oxidizing agents is utilized in qualitative tests such as the Benedict’s test and Fehling’s test to detect the presence of reducing sugars. In the food industry, the reducing properties of sugars play a role in processes like Maillard browning, contributing to the development of color, flavor, and aroma in baked goods and other foods. Non-reducing sugars, such as sucrose, serve as stable sweeteners and are often preferred in the preservation of fruits and vegetables due to their resistance to spoilage caused by microorganisms.
In biological systems, reducing sugars have essential roles. NADH and NADPH, two key coenzymes in cellular metabolism, contain reducing sugars in their structure, facilitating electron transfer reactions during energy production and biosynthesis. Non-reducing sugars, on the other hand, provide stable energy sources for cellular processes.
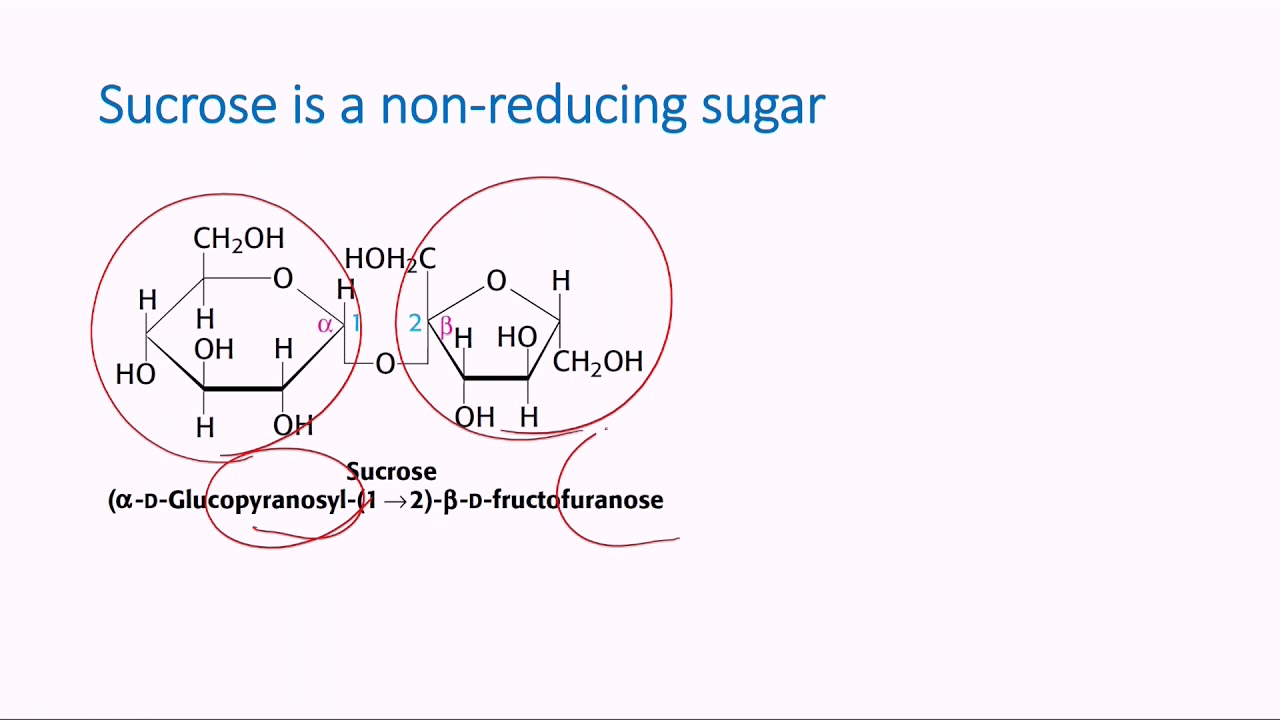
Image: webapi.bu.edu
Difference Between Reducing And Non Reducing Sugar
Conclusion
The distinction between reducing and non-reducing sugars lies in their molecular structure, specifically the presence or absence of a free aldehyde or keto group. Reducing sugars undergo redox reactions and can be detected using oxidizing agents like Benedict’s solution, while non-reducing sugars lack this ability. Understanding the difference between these two types of sugars is important in diverse fields, from food chemistry and nutrition to biochemistry and medicine. Whether in the production of food, the diagnosis of diseases, or the intricate workings of cellular metabolism, reducing and non-reducing sugars play crucial roles that shape our understanding of the world around us.